The cover of this issue of Cell is “Long-term histone lactylation connects metabolic and epigenetic rewiring in innate immune memory,” published by Professor Mihai G. Netea and Dr. Athanasios Ziogas of Radboud University Medical Center Nijmegen, the Netherlands.
Volume 188, Issue 11
Athanasios Ziogas1,2,15 athanasios.ziogas@radboudumc.nl ∙ Boris Novakovic3 ∙ Lorenzo Ventriglia1,4 ∙ Noriko Galang1,2 ∙ Kim A. Tran5 ∙ Wenchao Li6,7 ∙ Vasiliki Matzaraki1 ∙ Nienke van Unen6,7 ∙ Titus Schlüter1 ∙ Anaísa V. Ferreira1 ∙ Simone J.C.F.M. Moorlag1 ∙ Valerie A.C.M. Koeken1,8 ∙ Mthabisi Moyo1,2,9 ∙ Xiaolin Li1,2,9 ∙ Marijke P.A. Baltissen10 ∙ Joost H.A. Martens10 ∙ Yang Li6,7,11,12 ∙ Maziar Divangahi5 ∙ Leo A.B. Joosten1,13 ∙ Musa M. Mhlanga1,2,9 ∙ Mihai G. Netea1,14
Research Background
Trained immunity, a form of innate immune memory, has garnered increasing attention due to its ability to enhance responses to future challenges. This process is supported by epigenetic and metabolic reprogramming. Studies on individuals vaccinated with Bacillus Calmette–Guérin (BCG) have shown a correlation between lactate release and heightened cytokine responsiveness upon restimulation. Trained monocytes/macrophages exhibit a distinctive feature—histone H3 lysine 18 lactylation (H3K18la), which is predominantly located in distal regulatory regions. This lactylation is positively associated with active chromatin states and gene transcription, and it persists even after the initial training stimulus is removed. Importantly, H3K18la correlates with the expression of "trained" genes upon secondary stimulation.
Moreover, increased lactate production during the induction of trained immunity promotes the expression of pro-inflammatory cytokines, a process linked to histone lactylation. Polymorphisms in LDHA and EP300 genes influence trained immunity responses, and long-term histone lactylation can still be detected 90 days after BCG vaccination. These findings suggest that H3K18la serves as an epigenetic marker of innate immune memory.
Significance of the Study
This study represents a significant advance in the field of trained immunity. It reveals the intrinsic link between lactate release, histone lactylation, and enhanced cytokine responsiveness, as well as the underlying mechanisms involving epigenetic and metabolic reprogramming. These findings provide critical insights into the biological processes of trained immunity. The identified association between genetic polymorphisms in LDHA and EP300 and the modulation of trained immunity offers new avenues for understanding its genetic basis. Furthermore, the establishment of H3K18la as an epigenetic hallmark of innate immune memory introduces a novel biomarker for future studies, driving progress in this domain.
Future Perspectives
Future research can expand in several directions. One key area is the detailed investigation of the roles of lactate production, histone lactylation, and related genes in trained immunity, including signaling pathways and regulatory networks, to fully elucidate its molecular foundation. Another direction is the translation of these insights into clinical applications, such as the development of new vaccine strategies or immunotherapies, which may offer breakthroughs in disease prevention and treatment. In addition, understanding inter-individual variability in trained immune responses—and how this relates to genetic and environmental factors—will support the development of personalized medicine approaches. Long-term effects of trained immunity should also be continuously assessed, including its impact on overall immune function and potential links to chronic disease development, to comprehensively define its biological significance.
Cover design process
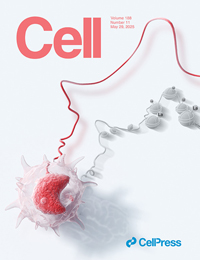 |
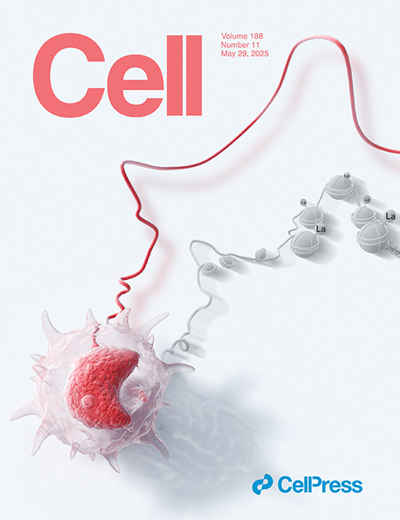
Innate immune cells can develop a memory-like state, resulting in more efficient secondary responses, a phenomenon known as trained immunity. This form of memory is encoded in innate immune cells at the epigenetic level. In this issue of Cell, Ziogas et al. demonstrate that lactate produced by monocytes can confer long-term epigenetic memory and regulate secondary responses through histone lactylation. The cover illustration presents a monocyte, with the cord symbolizing the dynamic and amplified secondary response characteristic of trained immunity, and histone lactylation in the background linked to the underlying epigenetic memory. |
Our hours
Beijing time: 9:00-18:00